CIC Exam Question 51
There are four cases of ventilator-associated pneumonia in a surgical intensive care unit with a total of 200 ventilator days and a census of 12 patients. Which of the following BEST expresses how this should be reported?
Correct Answer: B
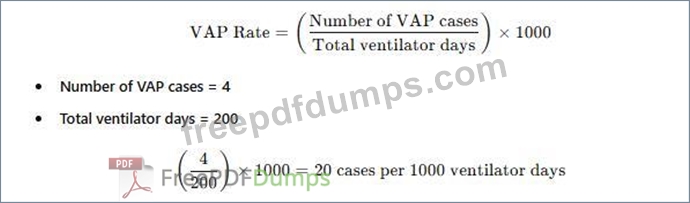
The standard way to report ventilator-associated pneumonia (VAP) rates is:
A white paper with black text AI-generated content may be incorrect.
Why the Other Options Are Incorrect?
* A. Ventilator-associated pneumonia rate of 2% - This does not use the correct denominator (ventilator days).
* C. Postoperative pneumonia rate of 6% in SICU patients - Not relevant, as the data focuses on VAP, not postoperative pneumonia.
* D. More information is needed regarding ventilator days per patient - The total ventilator days are already provided, so no additional data is required.
CBIC Infection Control Reference
APIC and NHSN recommend reporting VAP rates as cases per 1,000 ventilator days.
A white paper with black text AI-generated content may be incorrect.

Why the Other Options Are Incorrect?
* A. Ventilator-associated pneumonia rate of 2% - This does not use the correct denominator (ventilator days).
* C. Postoperative pneumonia rate of 6% in SICU patients - Not relevant, as the data focuses on VAP, not postoperative pneumonia.
* D. More information is needed regarding ventilator days per patient - The total ventilator days are already provided, so no additional data is required.
CBIC Infection Control Reference
APIC and NHSN recommend reporting VAP rates as cases per 1,000 ventilator days.
CIC Exam Question 52
Peripherally inserted central catheter (PICC)-associated bloodstream infections (BSIs) have been increasing over the past four months. Which of the following interventions is MOST likely to have contributed to the increase?
Correct Answer: C
Peripherally inserted central catheter (PICC)-associated bloodstream infections (BSIs) are a significant concern in healthcare settings, and identifying factors contributing to their increase is critical for infection prevention. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the
"Surveillance and Epidemiologic Investigation" and "Prevention and Control of Infectious Diseases" domains, which align with the Centers for Disease Control and Prevention (CDC) guidelines for preventing intravascular catheter-related infections. The question asks for the intervention most likely to have contributed to the rise in PICC-associated BSIs over four months, requiring an evaluation of each option based on evidence-based practices.
Option C, "Replacement of the intravenous administration sets every 72 hours," is the most likely contributor to the increase. The CDC's "Guidelines for the Prevention of Intravascular Catheter-Related Infections" (2017) recommend that intravenous administration sets (e.g., tubing for fluids or medications) be replaced no more frequently than every 72-96 hours unless clinically indicated (e.g., contamination or specific therapy requirements). Frequent replacement, such as every 72 hours as a routine practice, can introduce opportunities for contamination during the change process, especially if aseptic technique is not strictly followed. Studies cited in the CDC guidelines, including those by O'Grady et al. (2011), indicate that unnecessary manipulation of catheter systems increases the risk of introducing pathogens, potentially leading to BSIs. A change to a 72- hour replacement schedule, if not previously standard, could explain the observed increase over the past four months.
Option A, "Use of chlorhexidine skin antisepsis during insertion of the PICC," is a recommended practice to reduce BSIs. Chlorhexidine, particularly in a 2% chlorhexidine gluconate with 70% alcohol solution, is the preferred skin antiseptic for catheter insertion due to its broad-spectrum activity and residual effect, as supported by the CDC (2017). This intervention should decrease, not increase, infection rates, making it an unlikely contributor. Option B, "Daily bathing adult intensive care unit patients with chlorhexidine," is another evidence-based strategy to reduce healthcare-associated infections, including BSIs, by decolonizing the skin of pathogens like Staphylococcus aureus. The CDC and SHEA (Society for Healthcare Epidemiology of America) guidelines (2014) endorse chlorhexidine bathing in intensive care units, suggesting it should lower, not raise, BSI rates. Option D, "Use of a positive pressure device on the PICC," aims to prevent catheter occlusion and reduce the need for frequent flushing, which could theoretically decrease infection risk by minimizing manipulation. However, there is no strong evidence linking positive pressure devices to increased BSIs; if improperly used or maintained, they might contribute marginally, but this is less likely than the impact of frequent tubing changes.
The CBIC Practice Analysis (2022) and CDC guidelines highlight that deviations from optimal catheter maintenance practices, such as overly frequent administration set replacements, can increase infection risk.
Given the four-month timeframe and the focus on an intervention's potential negative impact, Option C stands out as the most plausible contributor due to the increased manipulation and contamination risk associated with routine 72-hour replacements.
References:
CBIC Practice Analysis, 2022.
CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2017.
O'Grady, N. P., et al. (2011). Guidelines for the Prevention of Intravascular Catheter-Related Infections.
Clinical Infectious Diseases.
SHEA Compendium, Strategies to Prevent Central Line-Associated Bloodstream Infections, 2014.
"Surveillance and Epidemiologic Investigation" and "Prevention and Control of Infectious Diseases" domains, which align with the Centers for Disease Control and Prevention (CDC) guidelines for preventing intravascular catheter-related infections. The question asks for the intervention most likely to have contributed to the rise in PICC-associated BSIs over four months, requiring an evaluation of each option based on evidence-based practices.
Option C, "Replacement of the intravenous administration sets every 72 hours," is the most likely contributor to the increase. The CDC's "Guidelines for the Prevention of Intravascular Catheter-Related Infections" (2017) recommend that intravenous administration sets (e.g., tubing for fluids or medications) be replaced no more frequently than every 72-96 hours unless clinically indicated (e.g., contamination or specific therapy requirements). Frequent replacement, such as every 72 hours as a routine practice, can introduce opportunities for contamination during the change process, especially if aseptic technique is not strictly followed. Studies cited in the CDC guidelines, including those by O'Grady et al. (2011), indicate that unnecessary manipulation of catheter systems increases the risk of introducing pathogens, potentially leading to BSIs. A change to a 72- hour replacement schedule, if not previously standard, could explain the observed increase over the past four months.
Option A, "Use of chlorhexidine skin antisepsis during insertion of the PICC," is a recommended practice to reduce BSIs. Chlorhexidine, particularly in a 2% chlorhexidine gluconate with 70% alcohol solution, is the preferred skin antiseptic for catheter insertion due to its broad-spectrum activity and residual effect, as supported by the CDC (2017). This intervention should decrease, not increase, infection rates, making it an unlikely contributor. Option B, "Daily bathing adult intensive care unit patients with chlorhexidine," is another evidence-based strategy to reduce healthcare-associated infections, including BSIs, by decolonizing the skin of pathogens like Staphylococcus aureus. The CDC and SHEA (Society for Healthcare Epidemiology of America) guidelines (2014) endorse chlorhexidine bathing in intensive care units, suggesting it should lower, not raise, BSI rates. Option D, "Use of a positive pressure device on the PICC," aims to prevent catheter occlusion and reduce the need for frequent flushing, which could theoretically decrease infection risk by minimizing manipulation. However, there is no strong evidence linking positive pressure devices to increased BSIs; if improperly used or maintained, they might contribute marginally, but this is less likely than the impact of frequent tubing changes.
The CBIC Practice Analysis (2022) and CDC guidelines highlight that deviations from optimal catheter maintenance practices, such as overly frequent administration set replacements, can increase infection risk.
Given the four-month timeframe and the focus on an intervention's potential negative impact, Option C stands out as the most plausible contributor due to the increased manipulation and contamination risk associated with routine 72-hour replacements.
References:
CBIC Practice Analysis, 2022.
CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2017.
O'Grady, N. P., et al. (2011). Guidelines for the Prevention of Intravascular Catheter-Related Infections.
Clinical Infectious Diseases.
SHEA Compendium, Strategies to Prevent Central Line-Associated Bloodstream Infections, 2014.
CIC Exam Question 53
Which of the following descriptions accurately describes a single-use medical device?
Correct Answer: D
The correct answer is D, "A device used one time on a patient during a procedure and then discarded," as this accurately describes a single-use medical device. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, a single-use device (SUD), also known as a disposable device, is labeled by the manufacturer for one-time use on a patient and is intended to be discarded afterward to prevent cross-contamination and ensure patient safety. This definition is consistent with regulations from the Food and Drug Administration (FDA), which designate SUDs as devices that should not be reprocessed or reused due to risks of infection, material degradation, or failure to restore sterility (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). Examples include certain syringes, catheters, and gloves, which are designed for single use to eliminate the risk of healthcare-associated infections (HAIs).
Option A (a device which can be used on a single patient) is too vague and could apply to both single-use and reusable devices, as reusable devices are also often used on a single patient per procedure before reprocessing.
Option B (a device that is sterilized and can be used again on the same patient) describes a reusable device, not a single-use device, as sterilization and reuse are not permitted for SUDs. Option C (a device used on a patient and reprocessed prior to being used again) refers to a reusable device that undergoes reprocessing (e.
g., sterilization), which is explicitly prohibited for SUDs under manufacturer and regulatory guidelines.
The focus on discarding after one use aligns with CBIC's emphasis on preventing infection through adherence to device labeling and safe reprocessing practices, ensuring that healthcare facilities avoid the risks associated with improper reuse of SUDs (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This practice is critical to maintaining a sterile and safe healthcare environment.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. FDA Guidance on Reprocessing of Single-Use Devices, 2016.
Option A (a device which can be used on a single patient) is too vague and could apply to both single-use and reusable devices, as reusable devices are also often used on a single patient per procedure before reprocessing.
Option B (a device that is sterilized and can be used again on the same patient) describes a reusable device, not a single-use device, as sterilization and reuse are not permitted for SUDs. Option C (a device used on a patient and reprocessed prior to being used again) refers to a reusable device that undergoes reprocessing (e.
g., sterilization), which is explicitly prohibited for SUDs under manufacturer and regulatory guidelines.
The focus on discarding after one use aligns with CBIC's emphasis on preventing infection through adherence to device labeling and safe reprocessing practices, ensuring that healthcare facilities avoid the risks associated with improper reuse of SUDs (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This practice is critical to maintaining a sterile and safe healthcare environment.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. FDA Guidance on Reprocessing of Single-Use Devices, 2016.
CIC Exam Question 54
At a facility with 10.000 employees. 5,000 are at risk for bloodbome pathogen exposure. Over the past five years, 100 of the 250 needlestick injuries involved exposure to bloodborne pathogens, and 2% of exposed employees seroconverted. How many employees became infected?
Correct Answer: B
To determine the number of employees who seroconverted (became infected) after a needlestick exposure, we use the given data:
* Total Needlestick Injuries: 250
* Needlestick Injuries Involving Bloodborne Pathogens: 100
* Seroconversion Rate: 2%
Calculation:
A black text with black numbers AI-generated content may be incorrect.
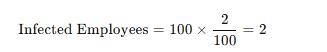
Why Other Options Are Incorrect:
* A. 1: Incorrect calculation; 2% of 100 is 2, not 1.
* C. 5: Overestimates the actual number of infections.
* D. 10: Exceeds the calculated value based on given data.
CBIC Infection Control References:
* APIC Text, "Occupational Exposure and Seroconversion Risks".
* APIC Text, "Bloodborne Pathogens and Needlestick Injury Prevention"
* Total Needlestick Injuries: 250
* Needlestick Injuries Involving Bloodborne Pathogens: 100
* Seroconversion Rate: 2%
Calculation:
A black text with black numbers AI-generated content may be incorrect.
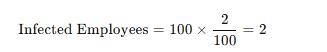
Why Other Options Are Incorrect:
* A. 1: Incorrect calculation; 2% of 100 is 2, not 1.
* C. 5: Overestimates the actual number of infections.
* D. 10: Exceeds the calculated value based on given data.
CBIC Infection Control References:
* APIC Text, "Occupational Exposure and Seroconversion Risks".
* APIC Text, "Bloodborne Pathogens and Needlestick Injury Prevention"
CIC Exam Question 55
Which of the following community-acquired infections has the greatest potential public health impact?
Correct Answer: A
The correct answer is A, "Cryptosporidium enteritis," as it has the greatest potential public health impact among the listed community-acquired infections. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the public health impact of an infection is determined by factors such as its transmissibility, severity, population at risk, and potential for outbreaks. Cryptosporidium enteritis, caused by the protozoan parasite Cryptosporidium, is a waterborne illness that spreads through contaminated water or food, leading to severe diarrhea, particularly in immunocompromised individuals. Its significant public health impact stems from its high transmissibility in community settings (e.g., via recreational water or daycare centers), the difficulty in eradicating the oocysts with standard chlorination, and the potential to cause large-scale outbreaks affecting vulnerable populations, such as children or the elderly (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.3 - Apply principles of epidemiology). This is exemplified by notable outbreaks, such as the 1993 Milwaukee outbreak affecting over 400,000 people.
Option B (Fifth disease, caused by parvovirus B-19) is a viral infection primarily affecting children, causing a mild rash and flu-like symptoms. While it can pose risks to pregnant women (e.g., fetal anemia), it is generally self-limiting and has limited community-wide transmission potential, reducing its public health impact. Option C (clostridial myositis, or gas gangrene, caused by Clostridium perfringens) is a severe but rare infection typically associated with traumatic wounds or surgery, with limited person-to-person spread, making its public health impact low due to its sporadic nature. Option D (cryptococcal meningitis, caused by Cryptococcus neoformans) primarily affects immunocompromised individuals (e.g., those with HIV/AIDS) and is not highly transmissible in the general community, confining its impact to specific at-risk groups rather than the broader population.
The selection of Cryptosporidium enteritis aligns with CBIC's focus on identifying infections with significant epidemiological implications, enabling infection preventionists to prioritize surveillance and control measures for diseases with high outbreak potential (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms). This is supported by CDC data highlighting waterborne pathogens as major public health concerns (CDC Parasites - Cryptosporidium, 2023).
References: CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.3 - Apply principles of epidemiology; Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms. CDC Parasites - Cryptosporidium, 2023.
Option B (Fifth disease, caused by parvovirus B-19) is a viral infection primarily affecting children, causing a mild rash and flu-like symptoms. While it can pose risks to pregnant women (e.g., fetal anemia), it is generally self-limiting and has limited community-wide transmission potential, reducing its public health impact. Option C (clostridial myositis, or gas gangrene, caused by Clostridium perfringens) is a severe but rare infection typically associated with traumatic wounds or surgery, with limited person-to-person spread, making its public health impact low due to its sporadic nature. Option D (cryptococcal meningitis, caused by Cryptococcus neoformans) primarily affects immunocompromised individuals (e.g., those with HIV/AIDS) and is not highly transmissible in the general community, confining its impact to specific at-risk groups rather than the broader population.
The selection of Cryptosporidium enteritis aligns with CBIC's focus on identifying infections with significant epidemiological implications, enabling infection preventionists to prioritize surveillance and control measures for diseases with high outbreak potential (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms). This is supported by CDC data highlighting waterborne pathogens as major public health concerns (CDC Parasites - Cryptosporidium, 2023).
References: CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.3 - Apply principles of epidemiology; Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms. CDC Parasites - Cryptosporidium, 2023.
- Latest Upload
- 124SAP.C_THR89_2505.v2025-12-16.q58
- 145Huawei.H12-821_V1.0.v2025-12-16.q153
- 115Juniper.JN0-750.v2025-12-16.q35
- 160Microsoft.SC-200.v2025-12-15.q150
- 136Fortinet.FCSS_EFW_AD-7.6.v2025-12-15.q26
- 150Microsoft.SC-300.v2025-12-15.q140
- 150Microsoft.MS-900.v2025-12-15.q191
- 138Avaya.78202T.v2025-12-14.q94
- 150EMC.D-PST-DY-23.v2025-12-14.q89
- 123HP.HPE0-S59.v2025-12-14.q35

