CIC Exam Question 66
An infection preventionist (IP) is informed of a measles outbreak in a nearby community. What is the IP's FIRST priority when working with Occupational Health?
Correct Answer: C
When an infection preventionist (IP) is informed of a measles outbreak in a nearby community, the immediate priority is to protect healthcare workers and patients from potential exposure, particularly in a healthcare setting where vulnerable populations are present. Working with Occupational Health, the IP must follow a structured approach to mitigate the risk of transmission, guided by principles from the Certification Board of Infection Control and Epidemiology (CBIC) and public health guidelines. Let's evaluate each option to determine the first priority:
* A. Isolate employees who have recently traveled to areas with measles outbreaks: Isolating employees who may have been exposed to measles during travel is an important infection control measure to prevent transmission within the facility. However, this action assumes that exposure has already occurred and requires identification of affected employees first. Without knowing the immunity status of the workforce, this step is reactive rather than preventive and cannot be the first priority.
* B. Reassign employees who are pregnant from caring for patients with suspected measles: Reassigning pregnant employees is a protective measure due to the severe risks measles poses to fetuses (e.g., congenital rubella syndrome risks, though measles itself is more about maternal complications). This action is specific to a subset of employees and depends on identifying patients with suspected measles, which may not yet be confirmed. It is a secondary step that follows assessing overall immunity and exposure risks, making it inappropriate as the first priority.
* C. Verify that employees in high-risk exposure areas of the facility have adequate immunity to measles:
Verifying immunity is the foundational step in preventing measles transmission in a healthcare setting.
Measles is highly contagious, and healthcare workers in high-risk areas (e.g., emergency departments, pediatric wards) are at increased risk of exposure. The CBIC and CDC recommend ensuring that all healthcare personnel have documented evidence of measles immunity (e.g., two doses of MMR vaccine, laboratory evidence of immunity, or prior infection) as a primary infection control strategy during outbreaks. This step allows the IP to identify vulnerable employees, implement targeted interventions, and comply with occupational health regulations. It is the most proactive and immediate priority when an outbreak is reported in the community.
* D. Set up a mandatory vaccination clinic in collaboration with Occupational Health and local public health partners: Establishing a vaccination clinic is a critical long-term strategy to increase immunity and control the outbreak. However, this requires planning, resource allocation, and coordination, which take time. It is a subsequent step that follows verifying immunity status to identify those who need vaccination. While important, it cannot be the first priority due to its logistical demands.
The first priority is C, as verifying immunity among employees in high-risk areas establishes a baseline to prevent transmission before reactive measures (e.g., isolation, reassignment) or broader interventions (e.g., vaccination clinics) are implemented. This aligns with CBIC's focus on proactive risk assessment and occupational health safety during infectious disease outbreaks, ensuring a rapid response to protect the healthcare workforce and patients.
:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain III:
Prevention and Control of Infectious Diseases, which prioritizes immunity verification during outbreaks.
CBIC Examination Content Outline, Domain IV: Environment of Care, which includes ensuring employee immunity as part of outbreak preparedness.
CDC Guidelines for Measles Prevention (2023), which recommend verifying healthcare worker immunity as the initial step during a measles outbreak.
* A. Isolate employees who have recently traveled to areas with measles outbreaks: Isolating employees who may have been exposed to measles during travel is an important infection control measure to prevent transmission within the facility. However, this action assumes that exposure has already occurred and requires identification of affected employees first. Without knowing the immunity status of the workforce, this step is reactive rather than preventive and cannot be the first priority.
* B. Reassign employees who are pregnant from caring for patients with suspected measles: Reassigning pregnant employees is a protective measure due to the severe risks measles poses to fetuses (e.g., congenital rubella syndrome risks, though measles itself is more about maternal complications). This action is specific to a subset of employees and depends on identifying patients with suspected measles, which may not yet be confirmed. It is a secondary step that follows assessing overall immunity and exposure risks, making it inappropriate as the first priority.
* C. Verify that employees in high-risk exposure areas of the facility have adequate immunity to measles:
Verifying immunity is the foundational step in preventing measles transmission in a healthcare setting.
Measles is highly contagious, and healthcare workers in high-risk areas (e.g., emergency departments, pediatric wards) are at increased risk of exposure. The CBIC and CDC recommend ensuring that all healthcare personnel have documented evidence of measles immunity (e.g., two doses of MMR vaccine, laboratory evidence of immunity, or prior infection) as a primary infection control strategy during outbreaks. This step allows the IP to identify vulnerable employees, implement targeted interventions, and comply with occupational health regulations. It is the most proactive and immediate priority when an outbreak is reported in the community.
* D. Set up a mandatory vaccination clinic in collaboration with Occupational Health and local public health partners: Establishing a vaccination clinic is a critical long-term strategy to increase immunity and control the outbreak. However, this requires planning, resource allocation, and coordination, which take time. It is a subsequent step that follows verifying immunity status to identify those who need vaccination. While important, it cannot be the first priority due to its logistical demands.
The first priority is C, as verifying immunity among employees in high-risk areas establishes a baseline to prevent transmission before reactive measures (e.g., isolation, reassignment) or broader interventions (e.g., vaccination clinics) are implemented. This aligns with CBIC's focus on proactive risk assessment and occupational health safety during infectious disease outbreaks, ensuring a rapid response to protect the healthcare workforce and patients.
:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain III:
Prevention and Control of Infectious Diseases, which prioritizes immunity verification during outbreaks.
CBIC Examination Content Outline, Domain IV: Environment of Care, which includes ensuring employee immunity as part of outbreak preparedness.
CDC Guidelines for Measles Prevention (2023), which recommend verifying healthcare worker immunity as the initial step during a measles outbreak.
CIC Exam Question 67
The cleaning and disinfection process that is appropriate for a particular surgical instrument depends on
Correct Answer: C
The correct answer is C, "the device manufacturer's written instructions for use," as this is the factor that determines the appropriate cleaning and disinfection process for a particular surgical instrument. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the reprocessing of surgical instruments must follow the specific instructions provided by the device manufacturer to ensure safety and efficacy. These instructions account for the instrument's material, design, and intended use, specifying the appropriate cleaning agents, disinfection methods, sterilization techniques, and contact times to prevent damage and ensure the elimination of pathogens (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This is also mandated by regulatory standards, such as those from the Food and Drug Administration (FDA) and the Association for the Advancement of Medical Instrumentation (AAMI), which require adherence to manufacturer guidelines to maintain device integrity and patient safety.
Option A (all surgical instruments are cleaned and sterilized in the same manner) is incorrect because different instruments have unique characteristics (e.g., materials like stainless steel vs. delicate optics), necessitating tailored reprocessing methods rather than a one-size-fits-all approach. Option B (instruments contaminated with blood must be bleach cleaned first) is a misconception; while blood contamination requires thorough cleaning, bleach is not universally appropriate and may damage certain instruments unless specified by the manufacturer. Option D (the policies of the sterile processing department) may guide internal procedures but must be based on and subordinate to the manufacturer's instructions to ensure compliance and effectiveness.
The emphasis on manufacturer instructions aligns with CBIC's focus on evidence-based reprocessing practices to prevent healthcare-associated infections (HAIs) and protect patients (CBIC Practice Analysis,
2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). Deviating from these guidelines can lead to inadequate sterilization or instrument damage, increasing infection risks.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
Option A (all surgical instruments are cleaned and sterilized in the same manner) is incorrect because different instruments have unique characteristics (e.g., materials like stainless steel vs. delicate optics), necessitating tailored reprocessing methods rather than a one-size-fits-all approach. Option B (instruments contaminated with blood must be bleach cleaned first) is a misconception; while blood contamination requires thorough cleaning, bleach is not universally appropriate and may damage certain instruments unless specified by the manufacturer. Option D (the policies of the sterile processing department) may guide internal procedures but must be based on and subordinate to the manufacturer's instructions to ensure compliance and effectiveness.
The emphasis on manufacturer instructions aligns with CBIC's focus on evidence-based reprocessing practices to prevent healthcare-associated infections (HAIs) and protect patients (CBIC Practice Analysis,
2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). Deviating from these guidelines can lead to inadequate sterilization or instrument damage, increasing infection risks.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
CIC Exam Question 68
A nurse exposed to pertussis develops a mild cough 14 days later. What is the recommended action?
Correct Answer: B
* The CDC recommends exclusion of healthcare workers with pertussis until completing at least five days of antibiotic therapy.
CBIC Infection Control References:
APIC-JCR Workbook, "Occupational Health Considerations," Chapter 10
CBIC Infection Control References:
APIC-JCR Workbook, "Occupational Health Considerations," Chapter 10
CIC Exam Question 69
Which of the following processes is essential for endoscope reprocessing?
Correct Answer: B
The correct answer is B, "Pre-cleaning, leak testing, and manual cleaning," as these processes are essential for endoscope reprocessing. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, proper reprocessing of endoscopes is critical to prevent healthcare-associated infections (HAIs), given their complex design and susceptibility to microbial contamination. The initial steps of pre-cleaning (removing gross debris at the point of use), leak testing (ensuring the endoscope's integrity to prevent fluid ingress), and manual cleaning (using enzymatic detergents to remove organic material) are foundational to the reprocessing cycle. These steps prepare the endoscope for high-level disinfection or sterilization by reducing bioburden and preventing damage, as outlined in standards such as AAMI ST91 (CBIC Practice Analysis,
2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). Failure at this stage can compromise subsequent disinfection, making it a non-negotiable component of the process.
Option A (intermediate level disinfection and contact time) is an important step but insufficient alone, as intermediate-level disinfection does not achieve the high-level disinfection required for semi-critical devices like endoscopes, which must eliminate all microorganisms except high levels of bacterial spores. Option C (inspection using a borescope and horizontal storage) includes valuable quality control (inspection) and storage practices, but these occur later in the process and are not essential initial steps; vertical storage is often preferred to prevent damage. Option D (leak testing, manual cleaning, and low level disinfection) includes two essential steps (leak testing and manual cleaning) but is inadequate because low-level disinfection does not meet the standard for endoscopes, which require high-level disinfection or sterilization.
The emphasis on pre-cleaning, leak testing, and manual cleaning aligns with CBIC's focus on adhering to evidence-based reprocessing protocols to ensure patient safety and prevent HAIs (CBIC Practice Analysis,
2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). These steps are mandated by guidelines to mitigate risks associated with endoscope use in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.4 - Implement environmental cleaning and disinfection protocols. AAMI ST91:2015, Flexible and semi-rigid endoscope processing in health care facilities.
2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). Failure at this stage can compromise subsequent disinfection, making it a non-negotiable component of the process.
Option A (intermediate level disinfection and contact time) is an important step but insufficient alone, as intermediate-level disinfection does not achieve the high-level disinfection required for semi-critical devices like endoscopes, which must eliminate all microorganisms except high levels of bacterial spores. Option C (inspection using a borescope and horizontal storage) includes valuable quality control (inspection) and storage practices, but these occur later in the process and are not essential initial steps; vertical storage is often preferred to prevent damage. Option D (leak testing, manual cleaning, and low level disinfection) includes two essential steps (leak testing and manual cleaning) but is inadequate because low-level disinfection does not meet the standard for endoscopes, which require high-level disinfection or sterilization.
The emphasis on pre-cleaning, leak testing, and manual cleaning aligns with CBIC's focus on adhering to evidence-based reprocessing protocols to ensure patient safety and prevent HAIs (CBIC Practice Analysis,
2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). These steps are mandated by guidelines to mitigate risks associated with endoscope use in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.4 - Implement environmental cleaning and disinfection protocols. AAMI ST91:2015, Flexible and semi-rigid endoscope processing in health care facilities.
CIC Exam Question 70
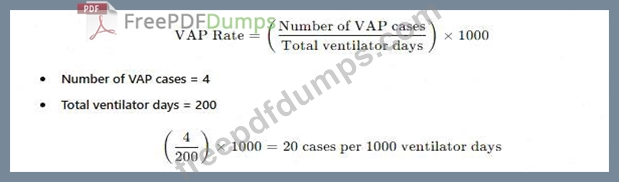
There are four cases of ventilator-associated pneumonia in a surgical intensive care unit with a total of 200 ventilator days and a census of 12 patients. Which of the following BEST expresses how this should be reported?
Correct Answer: B
The standard way to reportventilator-associated pneumonia (VAP) ratesis:
A white paper with black text AI-generated content may be incorrect.
Why the Other Options Are Incorrect?
* A. Ventilator-associated pneumonia rate of 2%- This does not use thecorrect denominator (ventilator days).
* C. Postoperative pneumonia rate of 6% in SICU patients-Not relevant, as the data focuses onVAP, not postoperative pneumonia.
* D. More information is needed regarding ventilator days per patient-The total ventilator days are already provided, so no additional data is required.
CBIC Infection Control Reference
APIC and NHSN recommend reporting VAP rates as cases per 1,000 ventilator days.
A white paper with black text AI-generated content may be incorrect.

Why the Other Options Are Incorrect?
* A. Ventilator-associated pneumonia rate of 2%- This does not use thecorrect denominator (ventilator days).
* C. Postoperative pneumonia rate of 6% in SICU patients-Not relevant, as the data focuses onVAP, not postoperative pneumonia.
* D. More information is needed regarding ventilator days per patient-The total ventilator days are already provided, so no additional data is required.
CBIC Infection Control Reference
APIC and NHSN recommend reporting VAP rates as cases per 1,000 ventilator days.
- Latest Upload
- 137CBIC.CIC.v2025-09-13.q75
- 122Cisco.700-841.v2025-09-13.q131
- 144SAP.C_ABAPD_2507.v2025-09-12.q56
- 127SAP.C_TS452_2022.v2025-09-12.q83
- 210ACSM.020-222.v2025-09-11.q48
- 160VMware.5V0-31.23.v2025-09-10.q73
- 132Oracle.1z0-915-1.v2025-09-10.q24
- 163Citrix.1Y0-231.v2025-09-10.q87
- 148SAP.C-THR85-2505.v2025-09-09.q29
- 181Adobe.AD0-E727.v2025-09-09.q76

